Table of Contents
Despite its critical nature, decision-making in research centers often relies on traditional methods, leading to inefficiencies and errors. This blog discusses how real-time, data-driven decision-making is transforming the clinical research landscape. It highlights the crucial role of dashboards in optimizing operational efficiency, improving patient outcomes, and streamlining the clinical trial execution process.
Why is data so important in clinical trial execution?
Clinical trial protocols are at the core of clinical research and are critical to bringing new drugs to market; their efficiency, accuracy, and precision are paramount in their execution.
Clinical research, in general, has undergone a radical transformation. Once managed in spreadsheets and filed away in folders, data is now the engine that drives innovation and efficiency in clinical trials. This transition from paper to digital platforms has enabled massive amounts of information to be collected, stored, and analyzed, generating a deeper understanding of trends. By using data analytics, clinical researchers can overcome complexity, reduce inefficiencies and medical errors, improve outcomes and coordination of care, and streamline every stage of the trial process.
Data-Driven Management in Research Centers
Operations in research centers are currently undergoing a major technological transformation. Decisions based on anecdotal evidence and intuition rapidly give way to data-driven decision-making. As data analytics redefines possible patient outcomes and operational efficiency, research centers must adopt a more sophisticated approach or risk being left behind. The abundance of data available in the digital age has brought with it an unprecedented opportunity for research centers to harness their full potential and drive critical decision-making processes.
Clinical trial execution has evolved from a data-rich but information-poor practice to an information-rich practice. Once cumbersome, data collection is leveraged to drive informed strategic decisions, marking a remarkable transformation in clinical trial management. This shift enables the identification of operational bottlenecks, thorough patient data analysis, and efficient resource allocation optimization. As a result, research centers can improve their performance and attract more patients to participate in their studies.
The Importance of Real-Time, Data-Driven Decision Making
Real-time, data-driven decision-making streamlines identifying issues, optimizing protocols, and improving study management. For example, by closely monitoring patient data, researchers can detect adverse events early and take corrective action, reducing participant risk and improving data quality. In addition, real-time data analysis makes it possible to identify patterns and trends that go unnoticed in traditional analysis, facilitating more informed and strategic decision-making.
Benefits of making data-driven decisions in clinical trials
- Increased efficiency: By automating processes and centralizing information, data management platforms can significantly reduce the time spent on administrative tasks, such as data collection and analysis.
- Cost reduction: Greater efficiency and process optimization translate into reduced costs associated with clinical trials. In addition, by attracting sponsors with solid data and reliable results, research centers, or networks of centers, can increase their income and secure more stable funding. As a curious fact, the duration of clinical trials has been reduced by 50%, and their success rate has improved by 10% with the incorporation of artificial intelligence into clinical trial data management.
- Better decision-making: Data allows researchers to identify the best strategies to recruit patients, optimize treatments, and evaluate the efficacy of new drugs. With accurate and up-to-date information, researchers can make more reliable and evidence-based decisions, which increases the probability of clinical trials’ success.
How to implement technology for data analysis in clinical trials
We have incorporated customizable dashboards into our Trial360 solution, which allows research sites to optimize operations and streamline clinical trials.
Trial360 Dashboards clearly and understandably present key metrics, from recruitment progress and enrollment rates to protocol compliance. Imagine instantly identifying delays in patient recruitment, tracking staff utilization, or visualizing trends in visit execution.
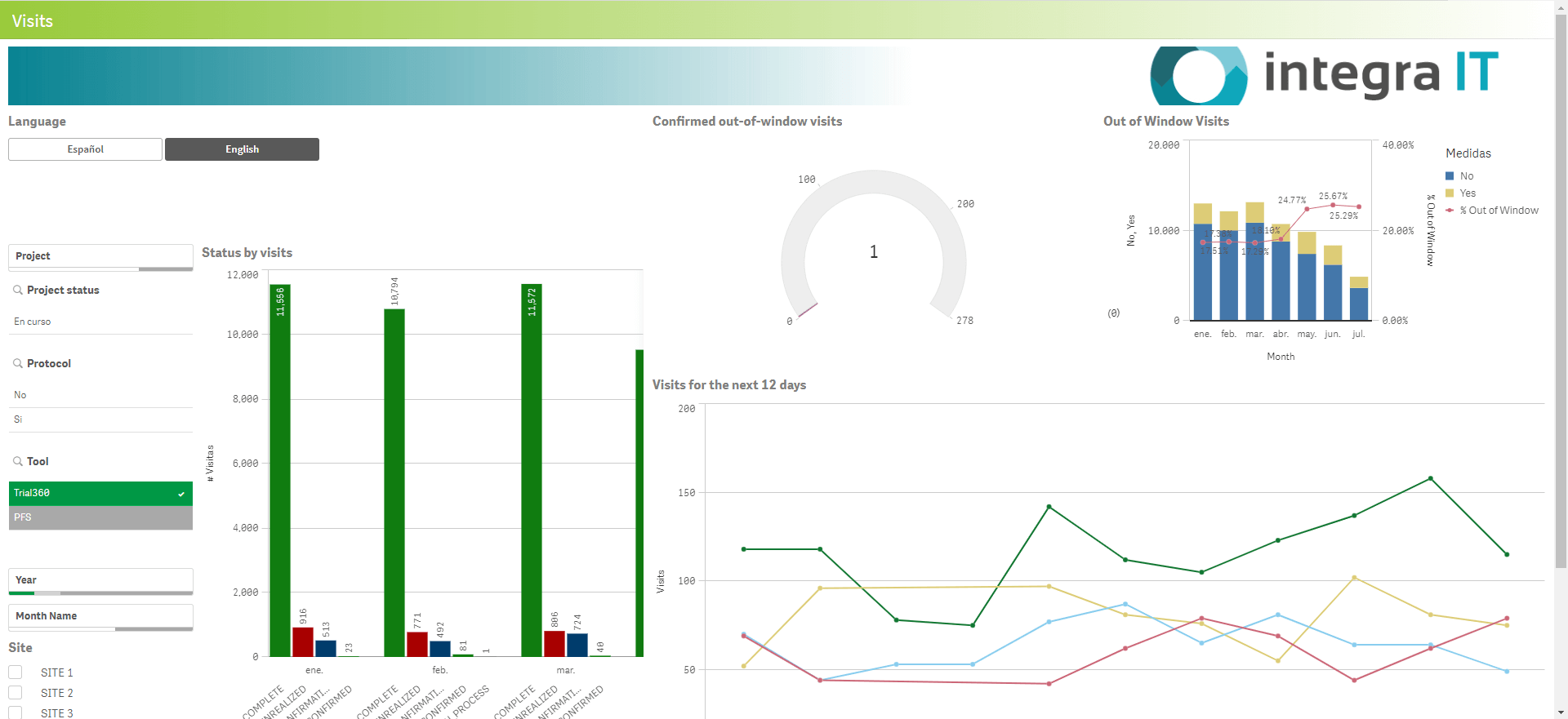
The Dashboards include performance indicators for your clinical trials and site operations such as finance, HR, marketing, procurement, and more. Trial360 is based on the Odoo platform, an integrated ERP software. In the My Dashboard module, you can find:
- Dynamic visualizations: Dashboards can be animated and updated in real-time, allowing you to visualize data more attractively and understandably.
- Flexibility: Dashboards adapt to different screen sizes and devices, offering an optimal user experience.
- Customization: Users can create their dashboards from scratch or use predefined templates and filter data by date for more specific information.
- Sharing and Exporting: Dashboards can be exported in different formats (Excel, CSV, PDF, PNG) to share with other users or integrate into other systems.
- Access Control: The tool allows you to set access permissions to the dashboards, ensuring that only authorized people can view and modify the information.
Trial360 dashboards outline critical points, facilitating faster decision-making, identifying trends, monitoring patient progress, and following the trial in real-time. This level of data visibility allows for quick identification of any issues that may arise and timely intervention and mitigation.
Dashboards also promote collaboration between the stakeholders involved in the clinical trial process. Providing a centralized platform where all team members can access the latest data and make informed decisions, leading to better overall trial outcomes.
Impact on Research Sites
Data-driven decision-making has already revolutionized several industries, and its impact on clinical trials is no exception. By leveraging tools like dashboards, research sites can streamline their processes and improve patient outcomes while remaining compliant with industry regulations.
Facing the Data-Driven Future
The transition to data-driven decision-making in clinical trials marks a notable advancement in clinical research. By leveraging all Trial360’s data analytics and dashboard capabilities, research sites can deftly navigate the complexities of trials with accuracy and efficiency. This improves patient outcomes and operational efficiency and puts these sites at the forefront of clinical research innovation.
Make informed decisions with real-time data. Discover Trial360’s boards today
References
- Towards Data-Driven Clinical Trial Planning and Strategy. (n.d.). Retrieved July 31, 2024, from https://www.appliedclinicaltrialsonline.com/view/towards-data-driven-clinical-trial-planning-and-strategy
- Las Tendencias de la Industria Farmacéutica 2024 – 2025 | Pharma (bismart.com)