Say goodbye to inflexible systems and paperwork. Clinical Research Associates can create customized reports, monitor data, and personalize workflows with TrialPal MoVi’s multilingual, user-friendly platform.

Say goodbye to inflexible systems and paperwork. Clinical Research Associates can create customized reports, monitor data, and personalize workflows with TrialPal MoVi’s multilingual, user-friendly platform.
TrialPal MoVi is a secure and validated web-based system that complies with FDA regulation 21 CFR Part 11, designed to accelerate the visit monitoring process from scheduling to report approval, during a clinical trial.
Create, examine, and approve monitoring visit reports with ease while keeping complete control.
Additionally, TrialPal MoVi Simplifies:
Submission/approval deadlines for follow-up monitoring visit reports and the resulting reports can be viewed for detailed monitoring of the trial’s status. This information can also be analyzed through customized dashboards, which allow the clinical trial’s indicators and metrics to be viewed in real time for timely decision-making.

Submission/approval deadlines for follow-up monitoring visit reports and the resulting reports can be viewed for detailed monitoring of the trial’s status. This information can also be analyzed through customized dashboards, which allow the clinical trial’s indicators and metrics to be viewed in real time for timely decision-making.
Click to see full image
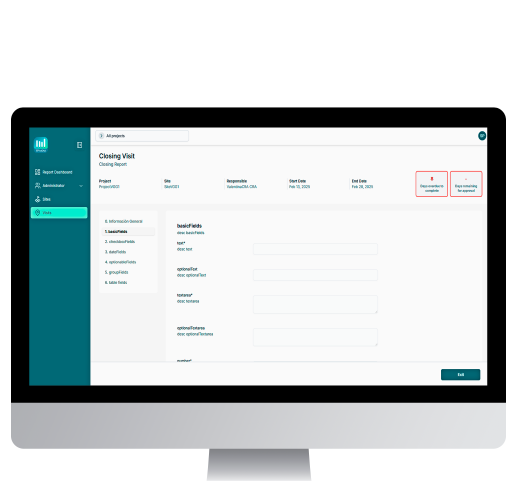
This module allows you to create and configure visits in the system. A customized report will be generated for each visit based on the clinical study’s requirements. Two users, who will assume the roles of Manager and Reviewer, will be associated to complete the reports and have them reviewed in a single, continuous visit flow.
Click to see full image
Click to see full image
Access dashboard panels with personalized metrics per study and get an overview of indicators like SAE reports SAE times, report type, and record dates of sponsor notifications in a graphic view.
This functionality is a time-saving tool for CROs and enables them to spend less time on transcription and paper tasks, so they can focus on analytics and efficiency.
In TrialPal MoVi, users with the CRA (Clinical Research Associates) role complete monitoring reports using a validation system that prevents certain formatting and typing errors. Additionally, users can save a report as a draft and continue it later without having to submit it for approval. They can also plan their monitoring activities and review study and research site information.
Project Managers (PMs) can manage multiple sites, review reports, and manage study information; specific personnel can access information to support planning and monitoring activities (e.g., CTAs) and perform oversight activities (e.g., line managers, quality department).
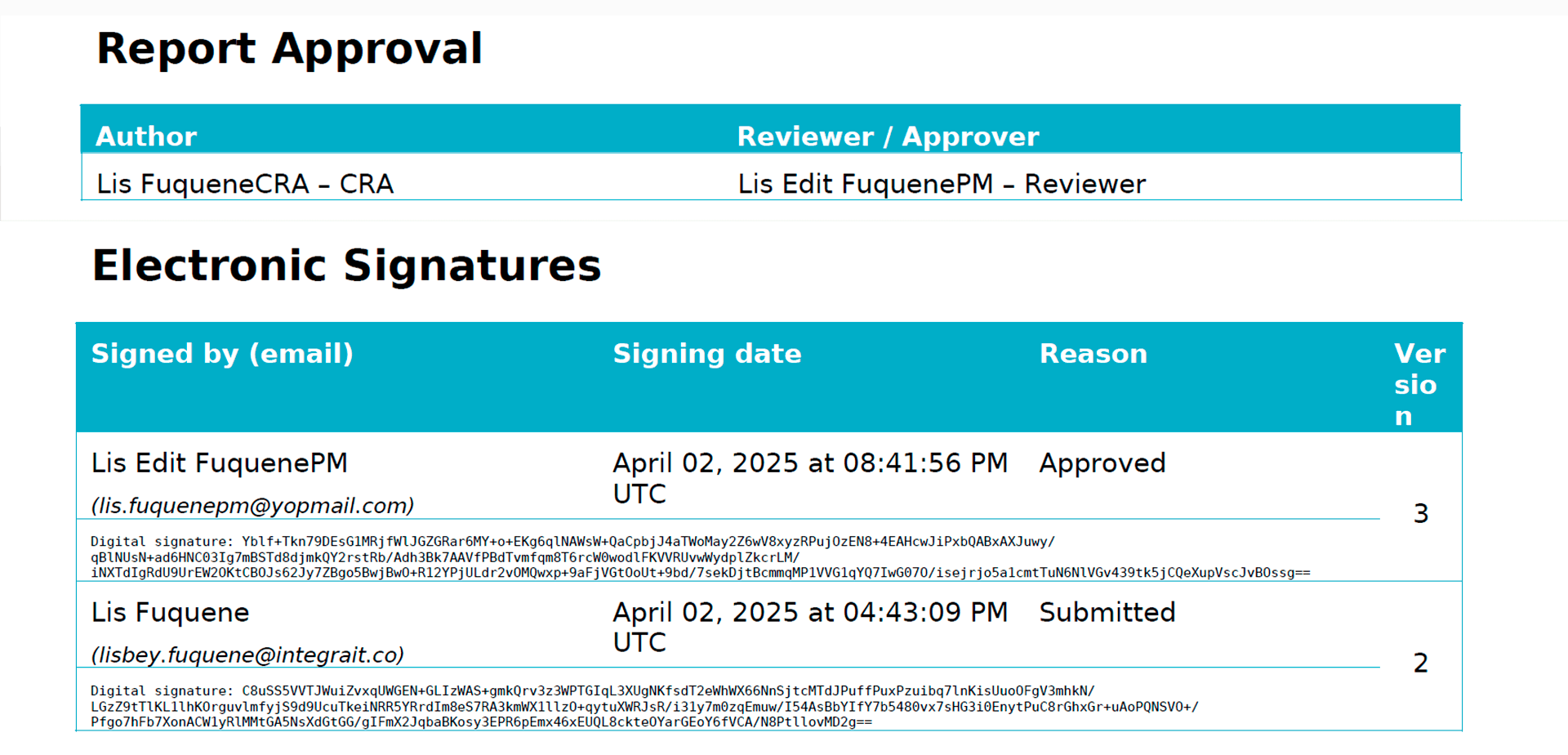
Within TrialPal MoVi, monitors and project managers certify that all processes are carried out in accordance with best practices by electronically signing reports.
Depending on the study requirements, TrialPal MoVi offers the following report types:
Schedule a meeting with us!
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |